Hydrogen's atomic number is 1 because all hydrogen atoms contain exactly one proton. Oct 15, 2020 Atomic number of hydrogen 3 letters.If you need help with Daily New York Times Crossword Answers, then you are here on the right place. Every day we will publish at our site the whole solution of the oldest crossword puzzle worldwide.
The atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, D). The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. (Atomic mass is also referred to as atomic weight, but the term 'mass' is more accurate.)
Atomic Number For Hydrogen Ion
For instance, it can be determined experimentally that neon consists of three isotopes: neon-20 (with 10 protons and 10 neutrons in its nucleus) with a mass of 19.992 amu and an abundance of 90.48%, neon-21 (with 10 protons and 11 neutrons) with a mass of 20.994 amu and an abundance of 0.27%, and neon-22 (with 10 protons and 12 neutrons) with a mass of 21.991 amu and an abundance of 9.25%. The average atomic mass of neon is thus:
| 0.9048 | × | 19.992 amu | = | 18.09 amu |
| 0.0027 | × | 20.994 amu | = | 0.057 amu |
| 0.0925 | × | 21.991 amu | = | 2.03 amu |
| 20.18 amu |

Atomic Number Of H
The atomic mass is useful in chemistry when it is paired with the mole concept: the atomic mass of an element, measured in amu, is the same as the mass in grams of one mole of an element. Thus, since the atomic mass of iron is 55.847 amu, one mole of iron atoms would weigh 55.847 grams. The same concept can be extended to ionic compounds and molecules. One formula unit of sodium chloride (NaCl) would weigh 58.44 amu (22.98977 amu for Na + 35.453 amu for Cl), so a mole of sodium chloride would weigh 58.44 grams. One molecule of water (H2O) would weigh 18.02 amu (2×1.00797 amu for H + 15.9994 amu for O), and a mole of water molecules would weigh 18.02 grams.
The original periodic table of the elements published by Dimitri Mendeleev in 1869 arranged the elements that were known at the time in order of increasing atomic weight, since this was prior to the discovery of the nucleus and the interior structure of the atom. The modern periodic table is arranged in order of increasing atomic number instead.
Atomic Number For Hydrogen 3
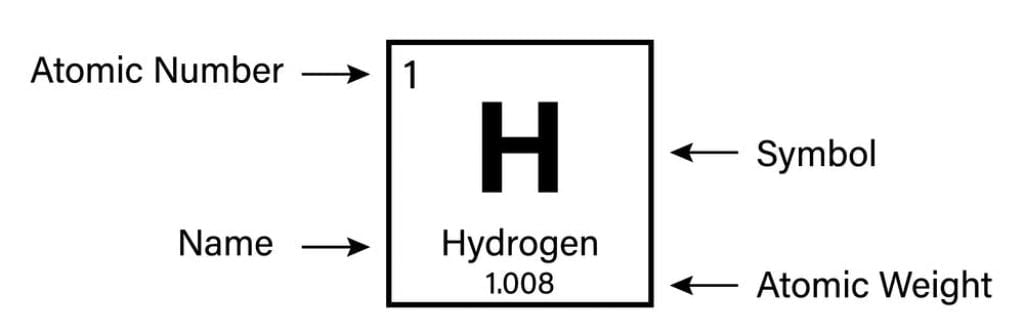
Atomic Number of Hydrogen is 1.

Chemical symbol for Hydrogen is H. Number of protons in Hydrogen is 1. Atomic weight of Hydrogen is 1.008 u or g/mol. Melting point of Hydrogen is -259,1 °C and its the boiling point is -252,9 °C.
» Boiling Point» Melting Point» Abundant» State at STP» Discovery YearAbout Hydrogen
Known as the most abundant and the lightest chemical element in our Universe, hydrogen is a type of gas without color and smell, which also has the lowest density of all gases. It is believed to be the first atom produced in our Universe after the Big Bang, and all other elements were further produced from hydrogen as a result of nuclear fusion. The name of the gas is formed from two Greek words, meaning water and forming, so this is the element which creates water. Since hydrogen is a part of water molecule, it is an absolutely essential chemical element for life, which can be found in all living bodies on our planet. It is extensively used in a large variety of industrial branches, from chemical industry (producing fertilizers, etc) to electronic (substance producing) and food industry, etc. One more very important point is: hydrogen is now seen as a source of clean eco-friendly fuel of the future, which will help the humanity to solve the problem of pollution and being gas/oil dependent.
Uses of Hydrogen
Hydrogen is mostly used in the petroleum and chemical industries. The most important use of hydrogen in the world is in ammonia manufacture for the fertilizer market. The other significant use of this chemical element is in fossil fuel processing. Today, liquid hydrogen is used as a primary fuel of the American space program by The National Aeronautics and Space Administration (NASA). Hydrogen is also used in various industrial fields such as metalworking and as a coolant in generators in power stations.
Compounds with Hydrogen
- H2O = Water (2 hydrogen, 1 oxygen)
- CH4 = Methane (1 carbon, 4 hydrogen)
- NH3 = Ammonia (1 nitrogen, 3 hydrogen)
- C6H12O6 = Glucose
- C9H8O4 = Aspirin
- NaHCO3 = Baking Soda
- C2H6O = Alcohol
- C12H22O11 = Sugar
- CH3COCH3 = Acetone
- C3H8 = Propane
- C2H4O2 = Acetic Acid
- HCI = Hydrochloric Acid
- C6H8O6 = Ascorbic Acid
- C3H6O3 = Lactic Acid
- H2S = Hydrogen Sulfide
- C2H4O2 = Vinegar
- C2H6O = Ethanol
- C4H10 = Butane
- C12H22O11 = Sucrose
- H2O2 = Hydrogen Peroxide
- NaOH = Sodium Hydroxide
- C6H6 = Benzene
- C10H16O = Camphor
- H2S = Hydrogen Sulfide
- CH3OH = Methanol
Properties of Hydrogen Element
| Atomic Number (Z) | 1 |
|---|---|
| Atomic Symbol | H |
| Group | 1 |
| Period | 1 |
| Atomic Weight | 1.008 u |
| Density | 0.00008988 g/cm3 |
| Melting Point (K) | 14.01 K |
| Melting Point (℃) | -259,1 °C |
| Boiling Point (K) | 20.28 K |
| Boiling Point (℃) | -252,9 °C |
| Heat Capacity | 14.304 J/g · K |
| Abundance | 1400 mg/kg |
| State at STP | Gas |
| Occurrence | Primordial |
| Description | Non-metal |
| Electronegativity (Pauling) χ | 2.2 |
| Ionization Energy (eV) | 13.59844 |
| Atomic Radius | 25pm |
| Covalent Radius | 38pm |
| Van der Waals Radius | 120 |
| Valence Electrons | 1 |
| Year of Discovery | 1766 |
| Discoverer | Cavendish |
What is the Boiling Point of Hydrogen?
Hydrogen boiling point is -252,9 °C. Boiling point of Hydrogen in Kelvin is 20.28 K.
What is the Melting Point of Hydrogen?
Hydrogen melting point is -259,1 °C. Melting point of Hydrogen in Kelvin is 14.01 K.
How Abundant is Hydrogen?
Atomic Number Hydrogen Isotopes
Abundant value of Hydrogen is 1400 mg/kg.
What is the State of Hydrogen at Standard Temperature and Pressure (STP)?
Atomic Number For Hydrogen 3
State of Hydrogen is Gas at standard temperature and pressure at 0℃ and one atmosphere pressure.
Atomic Number For Hydrogen Crossword
When was Hydrogen Discovered?
Hydrogen was discovered in 1766.
